CLINICAL TRANSLATIONAL SCIENCE (CTS) MODULE D2 Pilot PROJECTS –
REQUEST FOR APPLICATIONS
Institute for Integration of Medicine & Science / Clinical & Translational Science Award,
Deadline: January 17, 2025 (Letter of Intent)
Overview
The Institute for Integration of Medicine & Science (IIMS) is soliciting proposals for Clinical Translational Science (CTS) pilot project awards.
As defined by the National Center for Advancing Translational Sciences (NCATS), CTS is the field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process. Whereas clinical translational research (CTR) focuses on the specific case of a target or disease, CTS is focused on the general case that applies to any target or disease; advances in CTS are the focus of this RFA. A key tenet of CTS is to understand common causes of inefficiency and failure in translational research projects (e.g., incorrect predictions of the toxicity or efficacy of new drugs, lack of data interoperability, ineffective clinical trial recruitment). Many of these causes are the same across targets, diseases, and therapeutic areas; therefore, advances in CTS will increase the efficiency and effectiveness of CTR to enhance health, lengthen life, and reduce the burdens of illness and disability. Like any other science, CTS seeks to elucidate general operative principles to transform translation from an empirical, phenomenological process into a predictive science. Further details on CTS are described by Austin (Clinical Translational Science 2021;14:1629–1647, see DOI: 10.1111/cts.13055 ), as well as by Schneider et al. JCTS 8: e4, 1-8, 2023. We recommend that potential applicants link here to an annotated version of the Schneider article.
An example – for illustration only – may help clarify the distinction between CTR and CTS. An investigator who wishes to test whether a particular drug improves outcomes in diabetes will need to recruit sufficient underserved participants; this is a CTR problem and will be addressed from the standpoint of effectiveness for the drug’s effects and the diabetes community, using established recruitment methods. By contrast, an investigator who wishes to understand the fundamental underlying barriers to recruitment for clinical trials generally, and test an intervention directed at those hypothesized causes and mechanisms, is engaging in CTS. To test the hypothesis, the CTS investigator may choose a use case that may in fact be the same as that used by the CTR researcher – in this example a drug for diabetes – but the question to be answered is primarily whether the CTS innovation accomplishes full recruitment of the desired underserved population more effectively and efficiently.
The purpose of this RFA is to support CTS projects that address major roadblocks in clinical translational science. This program will not fund CTR pilot projects (please see the institutionally-supported CTR Pilot Project program if you wish to submit a CTR project).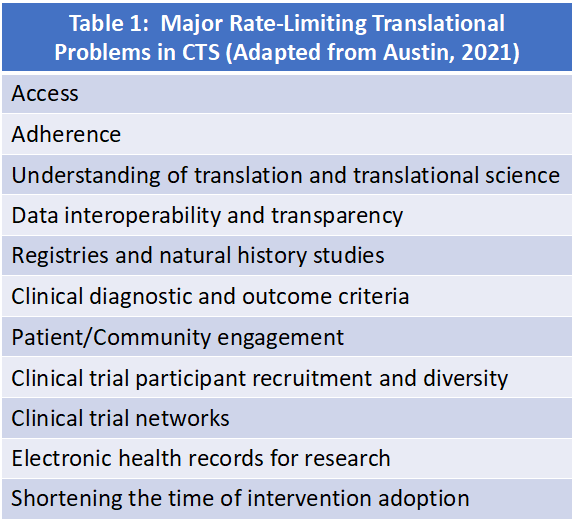 Table 1 summarizes many of these roadblocks and the successful project will address one of them.
Table 1 summarizes many of these roadblocks and the successful project will address one of them.
Amount and Term: Requests for funds of up to $50,000 will be considered for a 1-year project period. Funds are available to fund up to 6 projects. This program is supported by the UTHSCSA CTSA from the National Institutes of Health (NIH). Applicants must adhere fully to the guidelines and formats stipulated in this RFA, as non-complying applications may be administratively inactivated.
Eligibility
Applicants must hold faculty-level appointments at the University of Texas Health Science Center at San Antonio (UTHSCSA) or one of its CTSA partner/collaborator institutions (San Antonio Metropolitan Health District, San Antonio Military Health System, South Texas Veterans Health Care System, Texas Biomedical Research Institute, University Health, University of Texas San Antonio, University of Texas School of Public Health (San Antonio Regional Campus), University of Texas Austin). Pilot awards target in particular junior faculty with potential for career development impact. However, senior faculty, optimally in collaboration with a junior colleague, are eligible if the project is a novel departure from their currently funded programs.
Submission, terms, and conditions
An individual may submit no more than one project as Principal Investigator but may also serve as a Co-Investigator on one other project.
All applications (both letters of intent and full proposals) must be submitted through the Survey Monkey Apply platform (https://apply-uthscsa.smapply.io/) under the Principal Investigator’s account. The funding opportunity at that site is listed as: 2025 IIMS/CTSA CTS Pilot Projects
A required letter of intent (LOI) must be submitted by January 17, 2025 at 11:59 pm. The LOI should include the following:
- The title of the project
- Name and title of the PI and, if applicable Co-PI and Co-I
- Summary of the project (maximum 600 words)
- A list of 3 to 4 potential reviewers from UTHSCSA or CTSA partner institutions, but not from the same department or research group as the PI.
LOIs will be reviewed by IIMS/CTSA leadership to identify those having best alignment with IIMS programmatic goals. The CTSA will match the PIs of approved LOIs with a consultant from the CTSA CTS Design Studio who will provide feedback on study focus and design. This feedback will be provided to assist the preparation of a full application that addresses barriers pertinent to CTS. The deadline for receipt of the full application will be announced following evaluation of the LOIs. If you have any questions, please contact Cindy Castilleja (castillejac@uthscsa.edu) at 210-744-6446.
Awards will be made for a 1-year project period starting on or about July 1, 2025. Progress reports will be required six months (brief) and 12 months after the initiation of funding. In addition, a follow-up survey of related grants and publications will be solicited at 24, 36, and 48 months. Recipients who fail to submit timely and meaningful progress reports will be deemed ineligible for future funding cycles. For projects involving the use of human subjects, no expenditures will be permitted until IIMS is provided with a copy of the official letter of approval by the appropriate Institutional Review Board (IRB). Investigators are encouraged to submit IRB and IACUC protocols early in order to avoid significant delays in project initiation. In addition, CTSA-supported projects comprising either human or vertebrate animal research must be pre-approved by NCATS. Excessive delays in meeting these regulatory requirements may result in withdrawal of the award. Applicants must also be up to date on compliance with institutional research training and conflict of interest disclosure policies.
Budget and financial policies
The budget for these awards is up to $50,000 for 1 year. Facilities and Administrative (F&A, indirect cost) expenses will not be reimbursed. No salary support for the PI or faculty-level collaborators is allowed. Although the PI (and Co-PI/Co-I, if applicable) should be listed in the personnel section of the summary page, there is no minimum effort requirement. Salary (plus associated fringe benefits) may be requested for non-faculty support staff, including post-docs. Other allowable expenses include: equipment essential for the project (maximum $5,000, including computer hardware); PI or Co-PI domestic travel to relevant scientific meetings (maximum $2,500); consumable laboratory supplies; core facility fees at IIMS, Texas Biomed, UTSA, UT Austin or other sites; consultation fees (maximum $5,000); computer time; software; publication / presentation expenses; costs related to human subject enrollment and management (listed as “Patient Care Costs” on budget page); and other expenditures that can be justified as being essential for the completion of the project. Note that for projects making use of an IIMS Clinical Research Unit (CRU), a budget for these expenses must be developed in advance and submitted with the full application. For a quote, complete the Study Data Collection Form and contact Lisa Fleming – flemingl1@uthscsa.edu. Tuition expense is not an allowed budget item. Account management will be centralized within IIMS-CTSA, Texas Biomed, UTSA or UT with expenditures and encumbrances for UTHSCSA projects being committed as they are incurred. For projects supported at other CTSA partner institutions, funds will be disbursed at appropriate intervals, based on the receipt of invoices for budgeted expenditures.
Application requirements and format (for those invited to submit a full proposal)
Applications should be prepared using the templates provided and uploaded as a single .pdf document. Font size can be no smaller than 11 point, preferably Arial or Times New Roman. The font size for figures, figure legends, charts, and tables may be smaller, but must be clearly legible. Margins all-around should be at least 0.5”. Pages should be numbered sequentially. The length of the Research Plan (narrative with illustrations and tables included) is limited to 4 single-spaced pages. The organization of the proposal should be as follows:
- SurveyMonkey Apply website funding opportunity - 2025 IIMS/CTSA CTS Pilot Projects
- Link to additional application forms (here)
- The CTS project budget is included in the application and does not need to be attached separately
- Project summary (level appropriate for scientific peers in the field)
- Additional information regarding the project (included in the application and does not need to be attached separately) to include as appropriate:
- Past IIMS funding and list of grants obtained or applied for resulting from IIMS support
- Pending or planned scientifically related applications to other CTS project programs (e.g., Pepper Center, School of Medicine, SNPRC), including a summary of potential overlap
- Career development potential, if applicable
- Prospects and specific plans for outside funding
- Collaborative, interdisciplinary, or community engagement features, if applicable
- Description of how the CTS project will interact with existing programs of the IIMS-CTSA, Texas Biomed, UTSA, or other CTSA partners, as appropriate.
PDF Attachments (in this order)
- CRU budget, if applicable – (see above)
- Biographical sketch for PI (maximum 5 pages; for NIH template see https://grants.nih.gov/grants/forms/biosketch.htm)
- Biographical sketches for other key personnel (maximum 5 pages each)
- Research plan (maximum 4 pages)
- Hypothesis and specific aims
- Background and significance
- Preliminary data
- Work proposed (including statistical analysis, power calculations, pitfalls, alternatives)
- Literature citations (be selective, but no page limit – use continuation page)
- Letters of support (brief) from core directors or Research Imaging Institute are required (if applicable)
- Letters of collaboration (optional)
- Appendices are not allowed
- A UTHSCSA Certificate of Proposal (COP) is not required
Review process and criteria
Full applications will undergo a two-tiered system of review. The first phase, or scientific review, will be performed by the CTS Project Study Section, including appropriate content experts and representatives from CTSA resources and services and other CTSA partner organizations. Scientific merit will be scored by these reviewers based on the following criteria:
- Significance
- Novelty / innovation in addressing a CTS barrier (Table 1) – please specify the barrier addressed.
- Strength of the study protocol, including:
- Design
- Feasibility
- Preliminary data (to the extent available)
- Integration with ongoing research
- Quality of the investigative team
- Likelihood of future NIH or other competitive external funding
- Contribution to career development of the PI or other team members, if applicable
- Extent of meaningful interdisciplinary collaboration and / or community engagement
- Use and leveraging of IIMS-CTSA, Texas Biomed, UTSA, or partner resources (for example, core facilities, biobanking)
- Potential for ultimately improving health outcomes
- Protection of human subjects
A programmatic review will then be performed by the IIMS-CTSA leadership team for program relevance and potential public health impact, taking advantage of input from institutional and community partners. Funding decisions will be based on scientific merit, as well as programmatic considerations, such as breadth and depth of the overall pilot study portfolio, interactions among partners, community involvement, and balance among program areas and disciplines.
Special emphasis on health disparities
Projects that address health equity in the context of prevalent disparities in access to or quality of care among minority groups in our region (e.g., Hispanics, veterans, military personnel, rural residents) are of high priority.
Responsibilities of the Principal Investigator
The principal investigators of funded projects are required to:
- Abide by NIH rules and regulations
- Abide by IIMS-CTSA/Texas Biomed/UTSA, and/or CTSA partner policies and procedures
- Provide demographic information in a timely fashion, as required before expenditures can be authorized
- At the time of funding provide a complete list of other support, including other CTS project mechanisms, along with explanations of any potential scientific or budgetary overlap
- Provide a waiver of Facilities and Administrative (F&A) fees or Indirect Costs from your institution if you are a non-UTHSCSA investigator
- Submit complete and timely progress reports
- Acknowledge support from IIMS, Texas Biomed, and/or UTSA grants and institute/center funds in all publications and reports generated with CTS project resources (details to be provided at the time of funding)
2025 IIMS/CTSA CTS Projects
CLINICAL TRANSLATIONAL SCIENCE (CTS) MODULE D2 Pilot PROJECTS –
REQUEST FOR APPLICATIONS
Institute for Integration of Medicine & Science / Clinical & Translational Science Award,
Deadline: January 17, 2025 (Letter of Intent)
Overview
The Institute for Integration of Medicine & Science (IIMS) is soliciting proposals for Clinical Translational Science (CTS) pilot project awards.
As defined by the National Center for Advancing Translational Sciences (NCATS), CTS is the field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process. Whereas clinical translational research (CTR) focuses on the specific case of a target or disease, CTS is focused on the general case that applies to any target or disease; advances in CTS are the focus of this RFA. A key tenet of CTS is to understand common causes of inefficiency and failure in translational research projects (e.g., incorrect predictions of the toxicity or efficacy of new drugs, lack of data interoperability, ineffective clinical trial recruitment). Many of these causes are the same across targets, diseases, and therapeutic areas; therefore, advances in CTS will increase the efficiency and effectiveness of CTR to enhance health, lengthen life, and reduce the burdens of illness and disability. Like any other science, CTS seeks to elucidate general operative principles to transform translation from an empirical, phenomenological process into a predictive science. Further details on CTS are described by Austin (Clinical Translational Science 2021;14:1629–1647, see DOI: 10.1111/cts.13055 ), as well as by Schneider et al. JCTS 8: e4, 1-8, 2023. We recommend that potential applicants link here to an annotated version of the Schneider article.
An example – for illustration only – may help clarify the distinction between CTR and CTS. An investigator who wishes to test whether a particular drug improves outcomes in diabetes will need to recruit sufficient underserved participants; this is a CTR problem and will be addressed from the standpoint of effectiveness for the drug’s effects and the diabetes community, using established recruitment methods. By contrast, an investigator who wishes to understand the fundamental underlying barriers to recruitment for clinical trials generally, and test an intervention directed at those hypothesized causes and mechanisms, is engaging in CTS. To test the hypothesis, the CTS investigator may choose a use case that may in fact be the same as that used by the CTR researcher – in this example a drug for diabetes – but the question to be answered is primarily whether the CTS innovation accomplishes full recruitment of the desired underserved population more effectively and efficiently.
The purpose of this RFA is to support CTS projects that address major roadblocks in clinical translational science. This program will not fund CTR pilot projects (please see the institutionally-supported CTR Pilot Project program if you wish to submit a CTR project). Table 1 summarizes many of these roadblocks and the successful project will address one of them.
Table 1 summarizes many of these roadblocks and the successful project will address one of them.
Amount and Term: Requests for funds of up to $50,000 will be considered for a 1-year project period. Funds are available to fund up to 6 projects. This program is supported by the UTHSCSA CTSA from the National Institutes of Health (NIH). Applicants must adhere fully to the guidelines and formats stipulated in this RFA, as non-complying applications may be administratively inactivated.
Eligibility
Applicants must hold faculty-level appointments at the University of Texas Health Science Center at San Antonio (UTHSCSA) or one of its CTSA partner/collaborator institutions (San Antonio Metropolitan Health District, San Antonio Military Health System, South Texas Veterans Health Care System, Texas Biomedical Research Institute, University Health, University of Texas San Antonio, University of Texas School of Public Health (San Antonio Regional Campus), University of Texas Austin). Pilot awards target in particular junior faculty with potential for career development impact. However, senior faculty, optimally in collaboration with a junior colleague, are eligible if the project is a novel departure from their currently funded programs.
Submission, terms, and conditions
An individual may submit no more than one project as Principal Investigator but may also serve as a Co-Investigator on one other project.
All applications (both letters of intent and full proposals) must be submitted through the Survey Monkey Apply platform (https://apply-uthscsa.smapply.io/) under the Principal Investigator’s account. The funding opportunity at that site is listed as: 2025 IIMS/CTSA CTS Pilot Projects
A required letter of intent (LOI) must be submitted by January 17, 2025 at 11:59 pm. The LOI should include the following:
- The title of the project
- Name and title of the PI and, if applicable Co-PI and Co-I
- Summary of the project (maximum 600 words)
- A list of 3 to 4 potential reviewers from UTHSCSA or CTSA partner institutions, but not from the same department or research group as the PI.
LOIs will be reviewed by IIMS/CTSA leadership to identify those having best alignment with IIMS programmatic goals. The CTSA will match the PIs of approved LOIs with a consultant from the CTSA CTS Design Studio who will provide feedback on study focus and design. This feedback will be provided to assist the preparation of a full application that addresses barriers pertinent to CTS. The deadline for receipt of the full application will be announced following evaluation of the LOIs. If you have any questions, please contact Cindy Castilleja (castillejac@uthscsa.edu) at 210-744-6446.
Awards will be made for a 1-year project period starting on or about July 1, 2025. Progress reports will be required six months (brief) and 12 months after the initiation of funding. In addition, a follow-up survey of related grants and publications will be solicited at 24, 36, and 48 months. Recipients who fail to submit timely and meaningful progress reports will be deemed ineligible for future funding cycles. For projects involving the use of human subjects, no expenditures will be permitted until IIMS is provided with a copy of the official letter of approval by the appropriate Institutional Review Board (IRB). Investigators are encouraged to submit IRB and IACUC protocols early in order to avoid significant delays in project initiation. In addition, CTSA-supported projects comprising either human or vertebrate animal research must be pre-approved by NCATS. Excessive delays in meeting these regulatory requirements may result in withdrawal of the award. Applicants must also be up to date on compliance with institutional research training and conflict of interest disclosure policies.
Budget and financial policies
The budget for these awards is up to $50,000 for 1 year. Facilities and Administrative (F&A, indirect cost) expenses will not be reimbursed. No salary support for the PI or faculty-level collaborators is allowed. Although the PI (and Co-PI/Co-I, if applicable) should be listed in the personnel section of the summary page, there is no minimum effort requirement. Salary (plus associated fringe benefits) may be requested for non-faculty support staff, including post-docs. Other allowable expenses include: equipment essential for the project (maximum $5,000, including computer hardware); PI or Co-PI domestic travel to relevant scientific meetings (maximum $2,500); consumable laboratory supplies; core facility fees at IIMS, Texas Biomed, UTSA, UT Austin or other sites; consultation fees (maximum $5,000); computer time; software; publication / presentation expenses; costs related to human subject enrollment and management (listed as “Patient Care Costs” on budget page); and other expenditures that can be justified as being essential for the completion of the project. Note that for projects making use of an IIMS Clinical Research Unit (CRU), a budget for these expenses must be developed in advance and submitted with the full application. For a quote, complete the Study Data Collection Form and contact Lisa Fleming – flemingl1@uthscsa.edu. Tuition expense is not an allowed budget item. Account management will be centralized within IIMS-CTSA, Texas Biomed, UTSA or UT with expenditures and encumbrances for UTHSCSA projects being committed as they are incurred. For projects supported at other CTSA partner institutions, funds will be disbursed at appropriate intervals, based on the receipt of invoices for budgeted expenditures.
Application requirements and format (for those invited to submit a full proposal)
Applications should be prepared using the templates provided and uploaded as a single .pdf document. Font size can be no smaller than 11 point, preferably Arial or Times New Roman. The font size for figures, figure legends, charts, and tables may be smaller, but must be clearly legible. Margins all-around should be at least 0.5”. Pages should be numbered sequentially. The length of the Research Plan (narrative with illustrations and tables included) is limited to 4 single-spaced pages. The organization of the proposal should be as follows:
- SurveyMonkey Apply website funding opportunity - 2025 IIMS/CTSA CTS Pilot Projects
- Link to additional application forms (here)
- The CTS project budget is included in the application and does not need to be attached separately
- Project summary (level appropriate for scientific peers in the field)
- Additional information regarding the project (included in the application and does not need to be attached separately) to include as appropriate:
- Past IIMS funding and list of grants obtained or applied for resulting from IIMS support
- Pending or planned scientifically related applications to other CTS project programs (e.g., Pepper Center, School of Medicine, SNPRC), including a summary of potential overlap
- Career development potential, if applicable
- Prospects and specific plans for outside funding
- Collaborative, interdisciplinary, or community engagement features, if applicable
- Description of how the CTS project will interact with existing programs of the IIMS-CTSA, Texas Biomed, UTSA, or other CTSA partners, as appropriate.
PDF Attachments (in this order)
- CRU budget, if applicable – (see above)
- Biographical sketch for PI (maximum 5 pages; for NIH template see https://grants.nih.gov/grants/forms/biosketch.htm)
- Biographical sketches for other key personnel (maximum 5 pages each)
- Research plan (maximum 4 pages)
- Hypothesis and specific aims
- Background and significance
- Preliminary data
- Work proposed (including statistical analysis, power calculations, pitfalls, alternatives)
- Literature citations (be selective, but no page limit – use continuation page)
- Letters of support (brief) from core directors or Research Imaging Institute are required (if applicable)
- Letters of collaboration (optional)
- Appendices are not allowed
- A UTHSCSA Certificate of Proposal (COP) is not required
Review process and criteria
Full applications will undergo a two-tiered system of review. The first phase, or scientific review, will be performed by the CTS Project Study Section, including appropriate content experts and representatives from CTSA resources and services and other CTSA partner organizations. Scientific merit will be scored by these reviewers based on the following criteria:
- Significance
- Novelty / innovation in addressing a CTS barrier (Table 1) – please specify the barrier addressed.
- Strength of the study protocol, including:
- Design
- Feasibility
- Preliminary data (to the extent available)
- Integration with ongoing research
- Quality of the investigative team
- Likelihood of future NIH or other competitive external funding
- Contribution to career development of the PI or other team members, if applicable
- Extent of meaningful interdisciplinary collaboration and / or community engagement
- Use and leveraging of IIMS-CTSA, Texas Biomed, UTSA, or partner resources (for example, core facilities, biobanking)
- Potential for ultimately improving health outcomes
- Protection of human subjects
A programmatic review will then be performed by the IIMS-CTSA leadership team for program relevance and potential public health impact, taking advantage of input from institutional and community partners. Funding decisions will be based on scientific merit, as well as programmatic considerations, such as breadth and depth of the overall pilot study portfolio, interactions among partners, community involvement, and balance among program areas and disciplines.
Special emphasis on health disparities
Projects that address health equity in the context of prevalent disparities in access to or quality of care among minority groups in our region (e.g., Hispanics, veterans, military personnel, rural residents) are of high priority.
Responsibilities of the Principal Investigator
The principal investigators of funded projects are required to:
- Abide by NIH rules and regulations
- Abide by IIMS-CTSA/Texas Biomed/UTSA, and/or CTSA partner policies and procedures
- Provide demographic information in a timely fashion, as required before expenditures can be authorized
- At the time of funding provide a complete list of other support, including other CTS project mechanisms, along with explanations of any potential scientific or budgetary overlap
- Provide a waiver of Facilities and Administrative (F&A) fees or Indirect Costs from your institution if you are a non-UTHSCSA investigator
- Submit complete and timely progress reports
- Acknowledge support from IIMS, Texas Biomed, and/or UTSA grants and institute/center funds in all publications and reports generated with CTS project resources (details to be provided at the time of funding)
